Trace Elemental Partitioning on Clays Derived from Hydrothermal Muds of the El Tatio Geyser Field, Chile
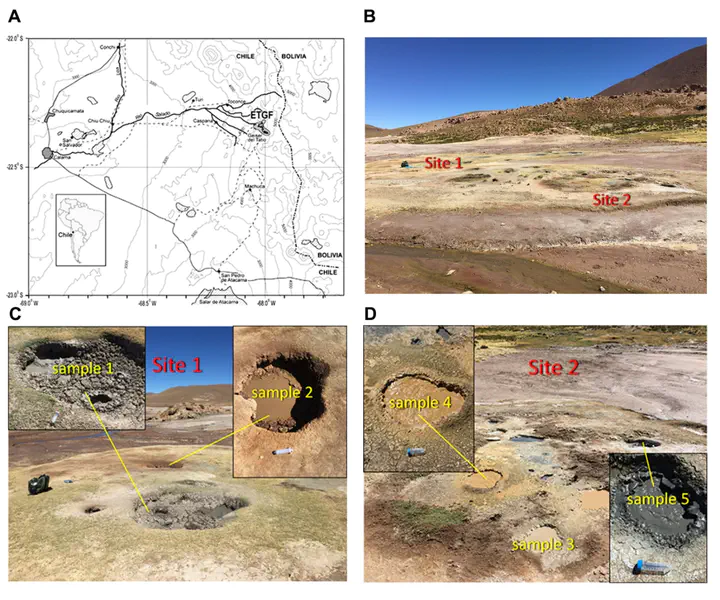 Sampled mud pots at the El Tatio Geyser Field, Chile.
Sampled mud pots at the El Tatio Geyser Field, Chile.
Abstract
Recent experimental studies have demonstrated that clay minerals (e.g., kaolinite, illite, and montmorillonite) have higher affinities for some trace elements under acidic versus alkaline conditions. This suggests that clays might be important vectors in the transport of trace elements from sites of acidic chemical weathering on land to marine depositional environments. To determine if clays behave similarly in nature, we collected water and mud (consisting 38.5-61.1% of kaolinite and montmorillonite) samples from boiling, low-pH, mud pools venting at the El Tatio Geyser Field (ETGF) in Chile. Based on elemental abundances in the aqueous/solid phases, we observed that mud samples collected from lower pH pools (e.g., pH=2.42, 3.55) have high concentration factors for anionic elements (e.g., P, As) but low concentration factors for cationic elements (e.g., Ca, Mn, Sr), while mud from higher pH pools (e.g., pH=4.87, 5.84) display the opposite trend. Acid-base leaching experiments further reveal that increasing solution pH (to reflect downstream transport) led to the release of As and P from the mud surfaces due to increasingly negative surface charge, while decreasing pH (to determine the effects of re-acidification) released Li, Ca, Co, Sr, Mo and Cd. Our study confirms previous experimental findings that demonstrate clay minerals can assemble a diverse inventory of trace elements during acid weathering (e.g., As) but then liberate them back into the aqueous phase as aqueous pH increases. Importantly, these observations provide a mechanism to account for the previous observations of regional As contamination in rivers downstream of the ETGF. This article is protected by copyright. All rights reserved.